Cleanroom Levels: Understanding the Different Classifications
- 2024-01-11
- View 12
Cleanrooms are specialized environments designed to minimize the presence of airborne particles and contaminants. These controlled spaces are crucial in industries such as pharmaceuticals, electronics manufacturing, biotechnology, and aerospace, where even the tiniest particle can have significant consequences Cleanrooms are classified into different levels based on their air cleanliness standards, and in this article, we will explore the various cleanroom levels and their specific requirements.
1. Introduction
Cleanrooms are designed to maintain specific levels of airborne particle cleanliness, temperature, humidity, and other parameters. They provide controlled environments that are essential for industries where product quality, safety, and reliability are of utmost importance Cleanroom classification ensures that the appropriate cleanliness standards are met and maintained.
2. What is a Cleanroom?
A cleanroom is a controlled environment that has a low level of airborne particles and other contaminants. It is constructed with specialized materials and equipped with advanced filtration and ventilation systems to maintain the desired cleanliness levels. Cleanrooms are typically sealed to prevent the entry of external contaminants and have strict protocols for personnel entry, gowning, and behavior.
3. Importance of Cleanroom Classification
Cleanroom classification is crucial for several reasons. Firstly, it helps establish a standardized framework for cleanliness levels, allowing organizations to set clear targets and ensure consistency across different cleanroom facilities. Secondly, it provides guidelines for the design, construction, operation, and maintenance of cleanrooms, enabling industries to meet specific regulatory requirements. Lastly, cleanroom classification assists in selecting the appropriate equipment, tools, and procedures necessary to achieve and maintain the desired cleanliness levels.
4. Cleanroom Levels and Classifications
Cleanrooms are classified into different levels based on various industry standards. The most commonly used cleanroom classification systems include ISO Classes, Federal Standard 209E Classes, and GMP (Good Manufacturing Practice) Classes.
4.1 ISO Classes
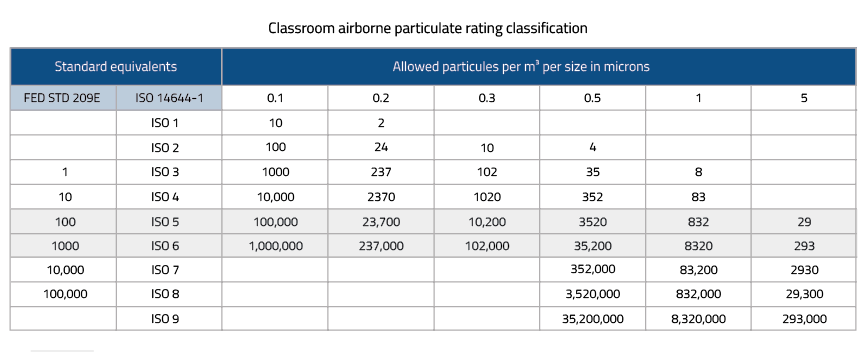
ISO (International Organization for Standardization) has developed a classification system known as ISO 14644-1. This standard defines the maximum allowable concentrations of airborne particles per cubic meter of air for different cleanroom classes. The ISO classification system provides a globally recognized standard for cleanroom cleanliness levels.
4.2 Federal Standard 209E Classes
The Federal Standard 209E classification system, which has been widely used in the past, defines cleanliness levels based on the number of particles per cubic foot of air. However, this classification system has been largely replaced by the ISO standards.
4.3 GMP Classes
GMP, or Good Manufacturing Practice, is a set of guidelines and regulations established by regulatory agencies such as the U.S. Food and Drug Administration (FDA) for industries involved in the production of pharmaceuticals, medical devices, and other healthcare-related products. GMP classes provide specific cleanliness requirements based on the intended use of the cleanroom and the products being manufactured.
5. Factors Affecting Cleanroom Levels
Several factors contribute to determining the cleanliness level of a cleanroom. These factors include airborne particle counts, airflow velocity, and air changes per hour (ACH).
5.1 Airborne Particle Counts
Airborne particle counts refer to the number and size of particles present in the cleanroom environment Different cleanroom levels have specified limits for particle counts, typically categorized by particle size ranges.
5.2 Airflow Velocity
Airflow velocity plays a crucial role in cleanrooms as it influences the movement and distribution of particles. Higher airflow velocities can help remove particles more efficiently, but they can also cause turbulence, which may result in particle resuspension.
5.3 Air Changes per Hour (ACH)
Air changes per hour (ACH) refer to the number of times the cleanroom's total air volume is replaced in one hour. A higher ACH means more frequent air exchanges, which can contribute to reducing particle concentration and maintaining cleanliness levels.
6. Cleanroom Level Guidelines
Different cleanroom levels have specific guidelines and requirements. Here are three common cleanroom classes based on the ISO standards:
6.1 ISO Class 1 - Ultra Clean
ISO Class 1 cleanrooms are the highest level of cleanliness, intended for applications requiring extremely low levels of airborne particles. These cleanrooms have strict protocols, including rigorous gowning procedures and airlocks, to maintain the ultra-clean environment necessary for industries like microelectronics and nanotechnology.
6.2 ISO Class 5 - Controlled Environment
ISO Class 5 cleanrooms, also known as "Class 100" cleanrooms under the Federal Standard 209E, have a controlled environment suitable for applications such as pharmaceutical manufacturing and biotechnology research. They have lower particle concentration limits compared to ISO Class 1, but still require meticulous cleanliness control.
6.3 ISO Class 8 - Controlled Environment
ISO Class 8 cleanrooms provide a controlled environment with slightly higher particle concentration limits. They are commonly used in industries such as medical device manufacturing and some research and development activities. While ISO Class 8 cleanrooms have higher particle limits, they still maintain a significantly cleaner environment compared to typical ambient conditions.
7. Cleanroom Level Conclusion
Cleanroom levels and classifications are essential in maintaining specific cleanliness standards and ensuring the quality and reliability of products in various industries. Understanding the different cleanroom levels, such as ISO classes, Federal Standard 209E classes, and GMP classes, enables organizations to establish and maintain controlled environments that meet their specific requirements. Factors like airborne particle counts, airflow velocity, and air changes per hour contribute to determining cleanroom levels. By adhering to appropriate cleanroom guidelines, industries can minimize the presence of contaminants and achieve optimal operational conditions.
Cleanroom Level FAQs
Q1. Can cleanroom levels be upgraded or downgraded based on changing requirements?
A1. Yes, cleanroom levels can be upgraded or downgraded based on the evolving needs of the industry or specific applications. However, any modifications to cleanroom classifications should be carefully planned and executed to ensure compliance with regulatory requirements and maintain product integrity.
Q2. Are there any specific industry regulations governing cleanroom classifications?
A2. Yes, different industries have specific regulations and guidelines governing cleanroom classifications. For example, the pharmaceutical industry follows GMP guidelines, while microelectronics may adhere to ISO standards. It is important for organizations to familiarize themselves with the relevant regulations and standards applicable to their industry.
Q3. Can cleanroom levels be customized for unique requirements?
A3. Cleanroom levels can be customized to meet unique requirements by considering factors such as particle size, cleanliness limits, airflow patterns, and filtration systems. Custom cleanroom designs can ensure that specific industry needs are met while maintaining appropriate cleanliness levels.
Q4. How often should cleanrooms be monitored for cleanliness levels?
A4. Cleanrooms should be regularly monitored for cleanliness levels to ensure compliance with the specified standards. The frequency of monitoring may vary based on industry regulations, internal quality assurance protocols, and the criticality of the products being manufactured.
Q5. What are some common methods for controlling particle contamination in cleanrooms?
A5. Common methods for controlling particle contamination in cleanrooms include high-efficiency particulate air (HEPA) filtration, laminar airflow systems, proper gowning procedures, frequent cleaning and disinfection, and regular maintenance of HVAC (Heating, Ventilation, and Air Conditioning) systems. These measures help minimize the introduction and accumulation of particles within the cleanroom environment.
Kwang Cleanroom is proud to offer examples of a variety of our cleanroom projects below. Dust-Free Workshop Company, Hospital Operating Theater Room, Cleanroom Technician, Cleanroom ISO 6, Clean Room for Pharmaceutical Industry, Sandwich Panel Cleanroom, Ultra Clean Room Systems.
