Navigating the Benefits and Functions of an ISO Class 8 Cleanroom
- 2024-01-18
- View 11
In the realm of precision manufacturing, pharmaceuticals, electronics, and biotechnology, maintaining the utmost levels of cleanliness and quality control is paramount. One integral player in achieving these standards is the ISO Class 8 cleanroom This controlled environment is designed to minimize particle contamination, ensuring that products and processes are conducted in a highly regulated and sterile setting. This article takes a comprehensive look at the features, benefits, and applications of an ISO Class 8 cleanroom.
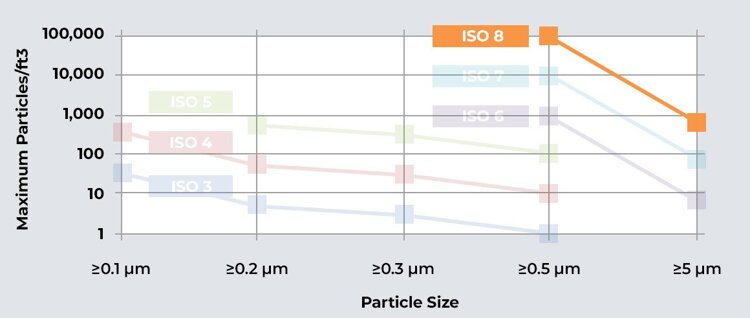
Decoding ISO Cleanroom Classes
Before we delve into the specifics of an ISO Class 8 cleanroom, it's essential to understand the classification system that defines the cleanliness levels in controlled environments.
The ISO Cleanroom Classification System
The ISO cleanroom classification system categorizes cleanrooms based on the number of airborne particles present per cubic meter. The lower the ISO classification number, the fewer particles are allowed. ISO Class 8 cleanrooms, therefore, allow a higher particle count compared to more stringent classifications.
Exploring ISO Class 8 Cleanrooms
ISO Class 8 cleanrooms are designed to maintain a controlled environment with a specific focus on industries that require precision, cleanliness, and reduced contamination risk.
Particle Control
In an ISO Class 8 cleanroom, the concentration of airborne particles is limited to 100,000 particles per cubic foot (approximately 3,520,000 particles per cubic meter). This level of particle control is vital for industries like electronics manufacturing, where even minor particle contamination can disrupt delicate processes.
Air Filtration
One of the foundational components of an ISO Class 8 cleanroom is the air filtration system. High-efficiency particulate air (HEPA) and ultra-low penetration air (ULPA) filters are employed to trap and remove particles from the air, contributing to the maintenance of a controlled environment.
Temperature and Humidity Control
ISO Class 8 cleanrooms often feature advanced systems for regulating temperature and humidity. These factors are critical in ensuring consistent product quality and preventing fluctuations that could negatively impact manufacturing processes.
Benefits of ISO Class 8 Cleanrooms
The adoption of an ISO Class 8 cleanroom offers a plethora of benefits to industries that prioritize precision and quality.
Reduced Contamination Risk
By controlling particle levels, ISO Class 8 cleanrooms significantly reduce the risk of contamination during manufacturing, assembly, and testing processes.
Enhanced Product Quality
Industries that require high-quality products, such as pharmaceuticals and medical devices, benefit from the controlled environment of ISO Class 8 cleanrooms, which minimizes defects and inconsistencies.
Regulatory Compliance
Many industries are subject to strict regulations and guidelines. Operating within an ISO Class 8 cleanroom helps businesses comply with these regulations, avoiding legal and financial complications.
Improved Research and Development
In research and development settings, where precision is key, ISO Class 8 cleanrooms provide the stable and controlled environment necessary for accurate experimentation and testing.
Applications of ISO Class 8 Cleanrooms
ISO Class 8 cleanrooms find applications across diverse industries:
Pharmaceuticals
Pharmaceutical manufacturing demands a sterile environment to ensure the safety and efficacy of medications. ISO Class 8 cleanrooms play a pivotal role in meeting these stringent requirements.
Electronics Manufacturing
The electronics industry relies on ISO Class 8 cleanrooms to prevent particle contamination that could lead to product malfunction or failure.
Biotechnology
In biotech research and development, ISO Class 8 cleanrooms facilitate the creation and testing of advanced products, such as genetically modified organisms and biopharmaceuticals.
ISO Class 8 Cleanroom Conclusion
In the intricate world of precision and high-quality manufacturing, the ISO Class 8 cleanroom stands as a testament to human innovation and dedication. Its controlled environment, particle control measures, and emphasis on cleanliness make it an indispensable asset for industries that demand precision and stringent quality control. As technology advances and industries evolve, the role of ISO Class 8 cleanrooms will remain pivotal in shaping products and processes that drive progress.
ISO Class 8 Cleanroom FAQs
1. What is the main purpose of an ISO Class 8 cleanroom?
The main purpose of an ISO Class 8 cleanroom is to provide a controlled environment with limited particle contamination, ensuring product quality, safety, and compliance with industry standards.
2. Are ISO Class 8 cleanrooms suitable for medical device manufacturing?
Yes, ISO Class 8 cleanrooms are suitable for medical device manufacturing, as they offer the required level of cleanliness and control to ensure the safety and efficacy of medical devices.
3. How often should air filters in an ISO Class 8 cleanroom be replaced?
The frequency of air filter replacement in an ISO Class 8 cleanroom depends on factors such as the type of filter, usage, and the level of contamination. Regular maintenance and monitoring are essential to determine the appropriate replacement schedule.
4. Can an existing facility be upgraded to an ISO Class 8 cleanroom?
Yes, existing facilities can be upgraded to meet the specifications of an ISO Class 8 cleanroom. However, it's essential to consult with experts to ensure proper design and implementation.
5. Are ISO Class 8 cleanrooms suitable for nanotechnology research?
Yes, ISO Class 8 cleanrooms can be suitable for nanotechnology research, especially when precise control over particle contamination is necessary for conducting accurate experiments and producing reliable results.
Kwang Cleanroom is proud to offer examples of a variety of our cleanroom projects below. Cleanroom ISO Class Standards, Biopharmaceutical Workshop, Bio-Cleanroom Design, Medical Purification Project, Vaccines Production Cleanrooms, Cleanroom Testing, Sterile Re-Packing Cleanrooms.
