ISO Clean Rooms: Ensuring Controlled Environments for Various Industries
- 2024-01-03
- View 9
Clean rooms are critical environments used in industries where contamination control is of utmost importance, such as pharmaceuticals, biotechnology, healthcare, electronics, and aerospace. These controlled environments provide the necessary conditions to safeguard sensitive processes, products, and equipment. The International Organization for Standardization (ISO) plays a vital role in establishing standards and guidelines for clean room design construction, and operation. In this article, we will explore the concept of ISO clean rooms their significance, classification system, design considerations, contamination control measures, applications, and the benefits they offer.
1. Understanding ISO Clean Rooms
ISO clean rooms are specialized facilities designed to maintain strict control over airborne particles, temperature, humidity, and other environmental factors. These controlled environments are constructed to minimize the introduction, generation, and retention of contaminants that can compromise the quality, safety, and efficacy of products and processes. ISO standards provide a framework for creating and maintaining clean rooms that meet specific cleanliness and performance requirements.
2. Importance of ISO Standards for Clean Rooms
ISO standards are essential for clean rooms as they ensure consistency, reliability, and compatibility across different industries and regions. They establish a common language and set of guidelines for designing, constructing, and operating clean rooms, facilitating effective communication and collaboration among stakeholders. Compliance with ISO standards helps industries achieve regulatory compliance, quality assurance, and customer satisfaction.
3. ISO Classification System
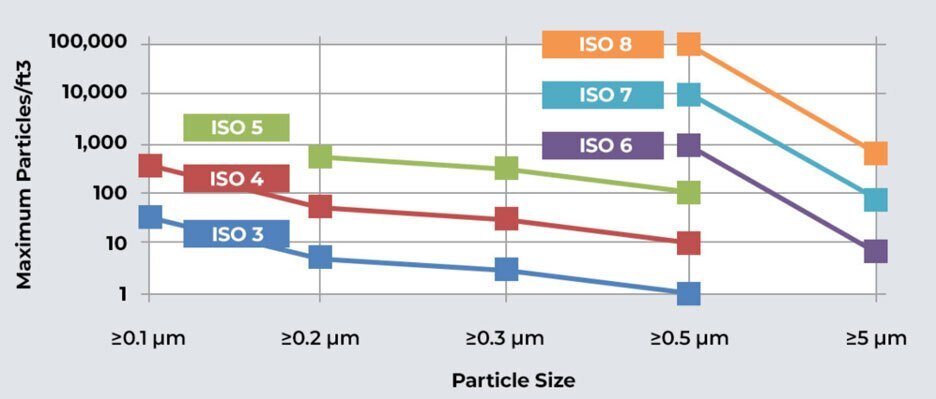
ISO clean rooms are classified based on the maximum allowable particle concentration in the air. The ISO classification system assigns a numerical value to represent the maximum particle size and concentration allowed per cubic meter of air. The commonly used ISO classes are ISO 1, ISO 2, ISO 3, ISO 4, ISO 5, ISO 6, ISO 7, and ISO 8, with ISO 1 being the most stringent and ISO 8 being the least stringent in terms of particle count.
4. Design and Construction of ISO Clean Rooms
The design and construction of ISO clean rooms involve several considerations to meet the desired cleanliness level. Key aspects include the selection of appropriate building materials, air filtration systems, airflow patterns, sealing mechanisms, and control of temperature and humidity. Clean room design professionals employ principles of laminar flow, air pressurization, and air recirculation to ensure consistent air quality and minimize the risk of contamination.
5. Controlling Contamination in ISO Clean Rooms
Contamination control is crucial in ISO clean rooms to maintain the desired cleanliness level. Various measures are implemented to control particle generation, including the use of high-efficiency particulate air (HEPA) filters, cleanable surfaces, controlled access points, and proper gowning protocols. Additionally, strict adherence to good manufacturing practices (GMP) and standard operating procedures (SOPs) helps minimize human-generated contamination.
6. Maintaining ISO Clean Room Standards
To sustain ISO clean room standards, regular monitoring, testing, and maintenance are essential. Routine activities include particle count monitoring, airflow velocity measurement, filter integrity testing, and surface cleanliness assessments. Deviations from the specified ISO class or performance criteria should trigger corrective actions to ensure ongoing compliance.
7. Applications of ISO Clean Rooms
ISO clean rooms find extensive applications across various industries. In pharmaceutical and biotechnology sectors, they are used for manufacturing sterile drugs, vaccines, and medical devices. In microelectronics and semiconductor industries, ISO clean rooms provide controlled environments for the fabrication of integrated circuits and electronic components. ISO clean rooms are also utilized in healthcare settings for surgeries, compounding sterile preparations, and conducting research.
8. Benefits of ISO Clean Rooms
Implementing ISO clean rooms offers several benefits to industries. They provide a controlled environment that protects sensitive processes and products from contamination. ISO clean rooms ensure consistent product quality, reduce the risk of product failures, and enhance customer confidence. They also contribute to employee safety by minimizing exposure to hazardous substances. Compliance with ISO standards demonstrates a commitment to quality and regulatory requirements, opening up opportunities for global market access.
9. Conclusion
ISO clean rooms are instrumental in maintaining contamination control and creating controlled environments in various industries. Adhering to ISO standards ensures consistency, reliability, and compatibility in clean room design, construction, and operation. ISO clean rooms offer numerous benefits, including enhanced product quality, regulatory compliance, and customer satisfaction. By implementing ISO clean rooms, industries can elevate their operations, protect sensitive processes, and deliver reliable and high-quality products.
10. Frequently Asked Questions
Q1: What does ISO stand for?
ISO stands for the International Organization for Standardization.
Q2: Are ISO clean rooms mandatory for all industries?
ISO clean rooms are not mandatory for all industries. However, industries that involve the manufacturing of sensitive products or processes, where contamination control is crucial, often choose to implement ISO clean rooms to ensure quality and compliance.
Q3: How often should ISO clean rooms be recertified?
The frequency of recertification for ISO clean rooms depends on various factors such as industry requirements, regulatory standards, and internal quality management systems. Typically, clean rooms are recertified annually or biennially to ensure continued compliance with cleanliness standards.
Q4: Can ISO clean rooms be modified or expanded after construction?
Yes, ISO clean rooms can be modified or expanded after construction. However, any modifications or expansions should be done while considering the impact on the clean room's performance, including its cleanliness level, airflow patterns, and overall integrity. It is crucial to consult with clean room design professionals and follow industry best practices when making modifications.
Q5: Are ISO clean rooms suitable for research and development activities?
Yes, ISO clean rooms are suitable for research and development activities that require controlled environments and contamination control. They provide an ideal setting for conducting experiments, developing prototypes, and testing new products in a controlled and sterile environment.
Kwang Cleanroom is proud to offer examples of a variety of our cleanroom projects below. Laminar Flow Clean Room, Clean Room Production, ISO Room, 100000 Level Purification Workshop, ISO 4 Cleanroom, Clean Room Level, Cleanroom ISO 6.
