Understanding ISO Cleanroom Standards: A Comprehensive Guide
- 2024-01-07
- View 8
In industries where precision, quality, and contamination control are paramount cleanrooms play a vital role. These controlled environments are designed to maintain low levels of airborne particles and microorganisms, ensuring the integrity of manufacturing processes, research activities, and other critical operations. To standardize cleanroom classification and regulation, the International Organization for Standardization (ISO) has established guidelines that are internationally recognized. In this article, we will delve into the significance of ISO cleanroom standards and how they contribute to maintaining the purity of controlled environments.
Introduction
Cleanrooms are critical for industries like semiconductor manufacturing, pharmaceuticals, biotechnology, and aerospace, where even the smallest particle can lead to defects or compromised results Cleanroom standards, particularly those set by the ISO, provide a systematic framework for creating, operating, and maintaining these controlled environments.
The Basics of Cleanroom Classification
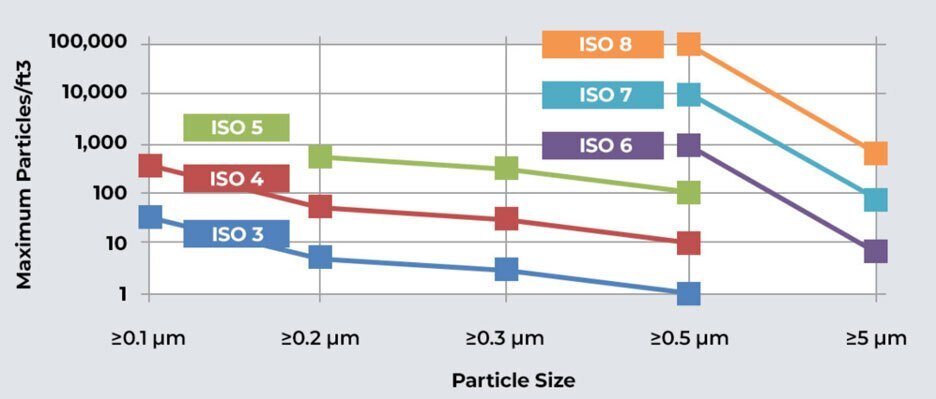
Cleanroom classification is based on the concentration of airborne particles within specific size ranges. ISO standards define the maximum allowable particle count for different classes of cleanrooms, ensuring uniformity and clarity across industries and geographical boundaries.
ISO Cleanroom Standards
ISO has established several standards related to cleanrooms, with ISO 14644 being a prominent series.
ISO 14644-1: Particle Count and Classification
ISO 14644-1 specifies the classification of air cleanliness by particle concentration. It defines cleanroom classes based on the number of particles per cubic meter at specified particle sizes.
ISO 14644-2: Monitoring and Testing
This part of the ISO 14644 series outlines procedures for monitoring and testing to demonstrate continued compliance with the established cleanroom classification. It covers aspects like instrument calibration, sampling methods, and data evaluation.
ISO Classes and Their Requirements
ISO cleanroom classes are numbered from 1 to 9, with lower numbers representing cleaner environments.
ISO Class 1-3: Ultra-Clean Environments
These classes demand the highest level of cleanliness, with stringent particle count requirements. They are crucial for industries like microelectronics, pharmaceuticals, and biotechnology.
ISO Class 4-9: Controlled Environments
These classes allow slightly higher particle counts and are suitable for industries like medical device manufacturing, optics, and food processing.
Implementing ISO Cleanroom Standards
Achieving and maintaining compliance with ISO cleanroom standards involves various aspects.
Design and Construction
Cleanrooms must be designed and constructed to meet specific cleanliness requirements. Factors like airflow, filtration, and material selection play a crucial role.
Operating Procedures and Protocols
Strict protocols for personnel behavior, gowning, and equipment usage are essential to prevent contamination. Regular monitoring and cleaning procedures are also integral to compliance.
Conclusion
ISO cleanroom standards provide a universal foundation for industries that depend on controlled environments. These standards ensure that the integrity of critical processes is maintained, ultimately leading to higher quality products and research outcomes.
FAQs
1. Why are ISO cleanroom standards important?
ISO cleanroom standards establish a consistent framework for maintaining cleanroom environments across industries, ensuring product quality and research integrity.
2. How are cleanrooms classified according to ISO standards?
Cleanrooms are classified based on the concentration of particles per cubic meter of air. ISO standards define different classes with specific particle count limits for various particle sizes.
3. Can cleanroom standards change over time?
Yes, cleanroom standards can evolve to incorporate advancements in technology and contamination control methods.
4. Are ISO cleanroom standards legally binding?
ISO standards themselves are not legally binding, but industries and regulatory agencies often adopt them as guidelines for compliance.
5. How do ISO cleanroom standards benefit the medical industry?
ISO cleanroom standards are crucial for the medical industry as they ensure the sterility and quality of medical devices, pharmaceuticals, and other healthcare products.
Kwang Cleanroom is proud to offer examples of a variety of our cleanroom projects below. Cleanroom Design and Build, Biopharmaceutical Cleanroom, Germfree Cleanroom, Class 10000 Clean Room, Design of Dust-Free Clean Room, Medical Device Clean Room, Hospital Operating Clean Room.
